
Once stigmatized as a party drug, MDMA is now central to groundbreaking clinical trials for depression treatment in Canada, representing a paradigm shift in mental health care and offering hope for those with treatment-resistant depression. This transformation of MDMA from recreational use to a therapeutic tool reflects our evolving understanding of mental health and the urgent need for effective treatments.
The Evolution of MDMA: From Recreational Use to Depression Treatment
MDMA’s journey from a recreational substance to a potential depression treatment highlights how our understanding of psychoactive compounds can change. MDMA’s potential in treating depression signifies a significant shift in mental health care.
Brief History of MDMA
MDMA, or 3,4-Methylenedioxymethamphetamine, was first synthesized in 1912 by Merck pharmaceuticals but initially received little attention. In the 1970s, some psychotherapists began using MDMA for its ability to enhance communication and introspection. However, its popularity in the 1980s as a recreational drug led to its classification as a Schedule I controlled substance in many countries, including Canada, which halted research for decades.
Shift in Perception: MDMA as a Therapeutic Tool
Interest in MDMA’s therapeutic potential resurfaced in the early 2000s, with organizations like the Multidisciplinary Association for Psychedelic Studies (MAPS) conducting rigorous clinical trials. These studies initially focused on MDMA-assisted psychotherapy for PTSD and showed promising results, challenging the long-held notion that MDMA was solely a dangerous party drug. This shift has opened new research avenues for MDMA’s potential in treating depression.
MDMA-Assisted Psychotherapy: A New Frontier in Depression Treatment
MDMA-assisted psychotherapy represents a groundbreaking approach, particularly for Canadians who haven’t found relief through conventional methods. This therapy combines MDMA’s unique neurochemical effects with structured psychotherapy sessions, offering a transformative experience for those with treatment-resistant depression.
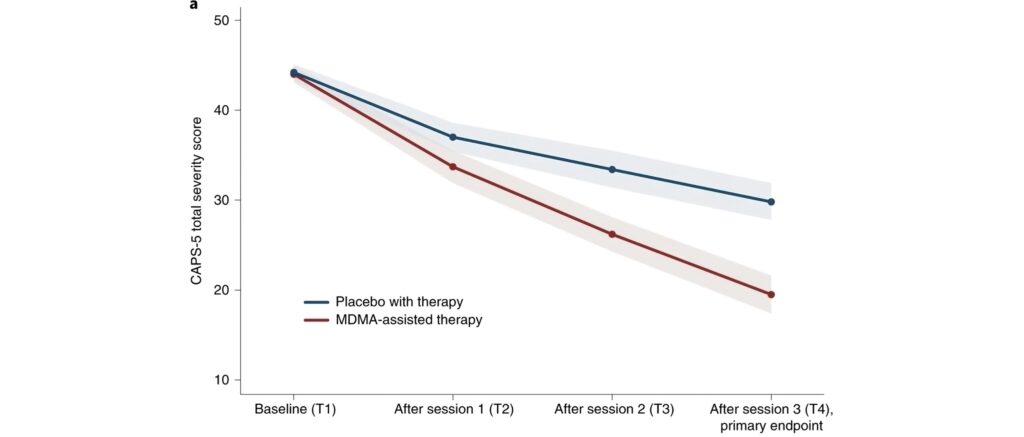
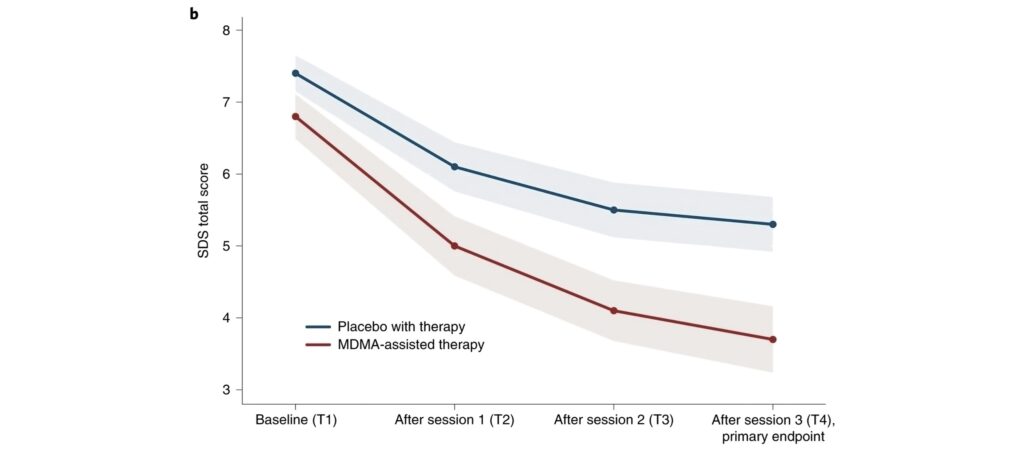
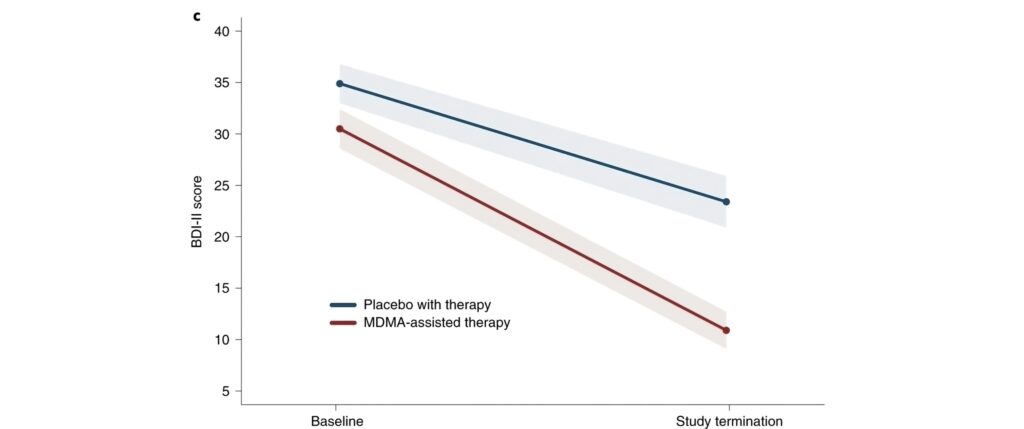
How MDMA Works in the Brain
MDMA primarily increases the release of neurotransmitters like serotonin, dopamine, and norepinephrine, enhancing mood and empathy. Importantly, it reduces activity in the amygdala (the fear center) and increases activity in the prefrontal cortex, allowing patients to confront traumatic memories or difficult emotions with less fear, breaking negative thought cycles often seen in depression.
Potential Benefits for Depression and PTSD
The benefits of MDMA-assisted psychotherapy for depression are significant. Patients report increased emotional openness and reduced anxiety during sessions, leading to more productive outcomes. While much research focuses on PTSD, the mechanisms by which MDMA helps—enhancing trust, reducing fear, and promoting emotional engagement—are also relevant for depression treatment, particularly for those with underlying trauma.
Current Status of MDMA Clinical Trials in Canada
Exciting developments are occurring in Canada regarding MDMA clinical trials. Several studies are underway, focusing primarily on treatment-resistant PTSD and depression, with trials conducted in Vancouver, Montreal, and Toronto. Preliminary results indicate significant reductions in depressive symptoms and improvements in overall well-being.
Overview of Ongoing Research
Currently, several MDMA clinical trials are underway across Canada, focusing primarily on treatment-resistant PTSD and depression. These studies are in various stages, from Phase 2 to Phase 3, and are being conducted at multiple sites including Vancouver, Montreal, and Toronto. The research aims to evaluate the safety and efficacy of MDMA-assisted psychotherapy in controlled, clinical settings.
One notable study is examining the effects of MDMA-assisted psychotherapy on individuals with treatment-resistant depression. This research is particularly promising for Canadians who haven’t found relief through conventional antidepressant medications or psychotherapy. The preliminary results are encouraging, showing significant reductions in depressive symptoms and improvements in overall well-being.
MAPS Initiatives
MAPS has been instrumental in MDMA research in Canada. Their Phase 3 clinical trials for PTSD have shown remarkable results, with many participants no longer meeting diagnostic criteria post-treatment. MAPS is also collaborating with Canadian researchers to expand studies on MDMA’s potential for other conditions related to depression.
The MDMA-Assisted Psychotherapy Process
The MDMA-assisted psychotherapy process is structured to maximize therapeutic benefits while ensuring patient safety. Understanding this process is crucial for Canadians seeking alternatives to traditional depression treatments.
Preparation and Screening
The preparation phase involves thorough medical and psychological evaluations to ensure patient suitability, building trust with therapists, and educating patients about MDMA’s effects.
MDMA Administration in a Therapeutic Setting
During sessions, patients receive a carefully measured dose of MDMA in a controlled environment, lasting 6-8 hours, with trained therapists present to provide support. Patients often experience increased emotional openness, allowing them to explore difficult emotions related to their depression.
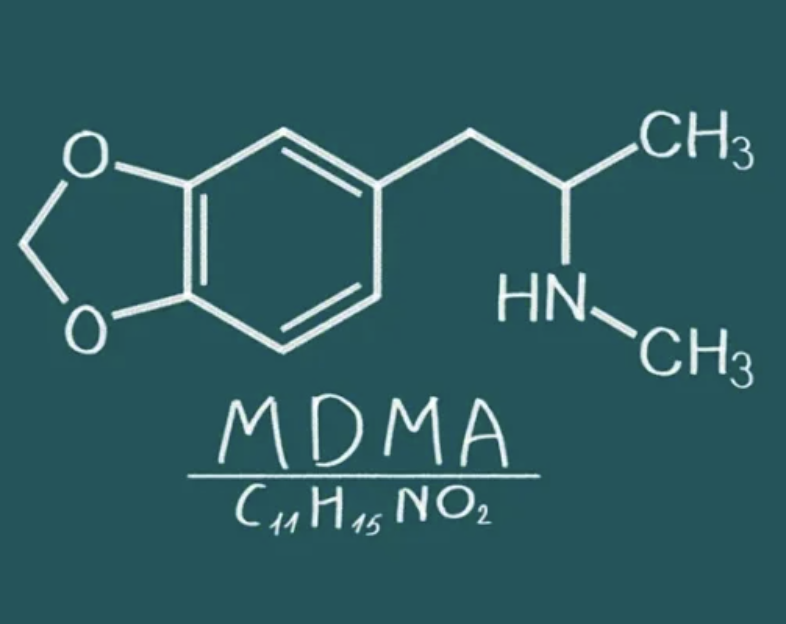
Integration Sessions and Follow-up Care
Integration therapy follows MDMA sessions, helping patients process experiences and apply insights to daily life. This ongoing support is crucial for sustaining therapeutic gains and is often more effective than traditional antidepressant treatments.
Comparing MDMA to Traditional Depression Treatments
As a doctor working extensively with patients suffering from depression, I’ve observed firsthand the limitations of traditional treatments. MDMA-assisted psychotherapy offers a novel approach that may address some of these shortcomings. For Canadians seeking alternatives to conventional therapies, understanding how MDMA compares to traditional treatments is crucial in making informed decisions about their mental health care.
Effectiveness vs. Antidepressant Medications
Traditional antidepressant medications, while effective for many, often come with drawbacks. They can take weeks to show effects, require daily dosing, and may cause side effects like weight gain or sexual dysfunction. In contrast, MDMA-assisted psychotherapy typically involves only a few doses, with effects often noticeable after the first session.
In clinical trials, MDMA has shown promising results, particularly for treatment-resistant cases. While antidepressants work by altering brain chemistry over time, MDMA appears to create a temporary state of increased emotional openness and reduced fear, allowing for more effective psychotherapy sessions. This combination of pharmacological and psychological effects may lead to more rapid and profound improvements in mood and cognitive function.
Potential Advantages for Treatment-Resistant Depression
For Canadians who haven’t responded well to traditional treatments, MDMA-assisted psychotherapy may help patients process underlying trauma or negative thought patterns that contribute to their depression, something that can be challenging with medication alone. The therapy’s focus on emotional processing and cognitive restructuring during MDMA sessions can lead to insights and breakthroughs that persist long after the drug’s immediate effects wear off.
Moreover, MDMA-assisted psychotherapy, combined with integration therapy, might require fewer sessions to achieve significant results compared to traditional psychotherapy. This could be particularly beneficial for those who have struggled with long-term depression and have found little relief from years of conventional treatments.
Safety Considerations and Potential Side Effects
While MDMA-assisted psychotherapy shows promise, understanding its safety profile is vital. In clinical settings, side effects are typically mild and short-lived, with most effects less severe than those associated with recreational use, thanks to controlled dosing.
Known Adverse Effects of MDMA
Common side effects include temporary increases in heart rate and blood pressure, jaw clenching, and mild anxiety. Long-term effects in therapeutic contexts are still being studied, but current research suggests that MDMA does not lead to cognitive impairments when used in controlled settings.
Importance of Controlled Administration and Professional Supervision
The safety of MDMA-assisted psychotherapy relies on controlled administration and professional oversight. Comprehensive support is provided before, during, and after treatment to ensure patient safety and the integrity of the study.
Legal Status and Future Outlook for MDMA Therapy in Canada
The regulatory landscape is shifting, reflecting growing recognition of MDMA’s therapeutic potential.
Current Regulatory Landscape
MDMA remains a Schedule I controlled substance in Canada, but Health Canada is increasingly open to psychedelic research. Several institutions are conducting MDMA clinical trials under strict regulations, paving the way for potential future therapeutic use.
Potential Timeline for FDA Approval and Canadian Accessibility
The timeline for MDMA approval in Canada is closely tied to developments in the United States. The FDA has granted MDMA Breakthrough Therapy designation for PTSD, expediting the review process. If current Phase 3 trials maintain positive results, FDA approval for MDMA-assisted psychotherapy for PTSD could come as early as 2023.
Following potential FDA approval, Health Canada typically conducts its own review process. This could mean MDMA-assisted psychotherapy might become legally available in Canada within 1-2 years after U.S. approval. However, it’s important to note that initial approval will likely be for PTSD treatment. Approval for depression treatment may take longer, as specific trials for this indication are still in earlier stages.
How Canadians Can Participate in MDMA Clinical Trials
Being involved in MDMA research as a doctor, I’m often asked by Canadians struggling with depression how they can participate in clinical trials. For those considering MDMA-assisted psychotherapy, understanding how to find and participate in these trials is crucial.
Finding Active MDMA Clinical Trials
The first step in participating in MDMA clinical trials is locating active studies. In Canada, several resources can help you find ongoing trials. The Canadian Institutes of Health Research (CIHR) maintains a database of clinical trials, including those involving MDMA for depression treatment. Additionally, the website ClinicalTrials.gov provides a comprehensive list of studies worldwide, including those in Canada.
Another valuable resource is the Multidisciplinary Association for Psychedelic Studies (MAPS) website. MAPS is at the forefront of MDMA research and regularly updates information about ongoing and upcoming trials. Local universities and research hospitals in major Canadian cities like Toronto, Vancouver, and Montreal are also key sites for MDMA research, and their websites often list current studies.
Eligibility Criteria and Application Process
Eligibility criteria typically include age range, specific diagnosis, and absence of certain medical conditions. Most trials exclude individuals with a history of psychosis or certain cardiovascular issues. It’s important to note that having tried traditional treatments without success is often a prerequisite for MDMA trials focused on treatment-resistant depression.
The application process usually begins with a preliminary screening, often through a phone call or online questionnaire. If you meet the initial criteria, you’ll likely be invited for a more comprehensive in-person assessment. This may involve medical examinations, psychological evaluations, and detailed discussions about your medical history. It’s a thorough process designed to ensure your safety and the study’s integrity. Remember, being honest and transparent during this process is crucial for your safety and the validity of the research.
The Broader Context: Psychedelic Therapy in Canada
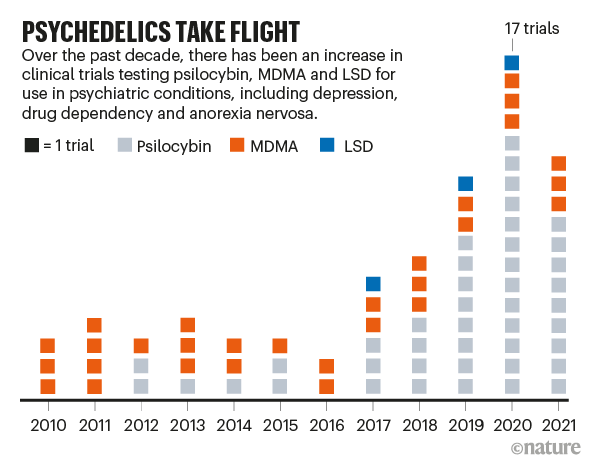
The exploration of MDMA for depression is part of a broader movement towards psychedelic therapy. Other compounds like psilocybin and Ibogaine are also being investigated for their potential in treating depression.
Other Psychedelic Drugs in Clinical Trials
While MDMA is at the forefront of psychedelic research for depression, it’s not the only compound under investigation. Psilocybin, the active ingredient in magic mushrooms, is showing remarkable promise in clinical trials for depression treatment. Studies have shown that psilocybin-assisted therapy can lead to significant reductions in depressive symptoms, often after just one or two sessions.
One dissociative anesthetic, while not a classic psychedelic, is another substance gaining traction in depression treatment. Some clinics in Canada are already offering psychedelic-assisted therapy for treatment-resistant depression, providing rapid relief for some patients who haven’t responded to traditional antidepressants. RTMS has also proven fruitful for treatment-resistant depression, which you can learn more about here.
The Future of Mental Health Treatment in Canada
The growing body of research on psychedelics is reshaping mental health treatment, offering new possibilities for those struggling with depression. While still in the research phase, these therapies could become important tools in mental health care, providing hope for many Canadians who have found little relief from traditional options.
Is MDMA Therapy available in Canada? It is at Field Trip Health! Learn more or book your discovery call with our health practitioners here today.
Field Trip Health Toronto offers additional alternative treatments for treatment-resistant depression, anxiety, PTSD, and other mental health disorders such as Stellate Ganglion Block therapy, MDMA therapy, Psilocybin therapy, Neurofeedback therapy, and repetitive Transcranial Magnetic Stimulation (rTMS)
About the Author

Dr. Mario Nucci MD CCFP is a licensed Family Physician with a passion for mental health and the development of new therapies. He is actively engaged in research with a faculty associate professorship at Northern Ontario School of Medicine, and research collaborations with the University of Ottawa, University of Calgary, Lakehead University, Concordia University and Vancouver Island University.
Dr. Nucci is the founder of Bay and Algoma Health Centre in 2019, a walk-in and addiction medicine clinic. He founded the Canadian Centre for Psychedelic Healing in 2019, now operating as Field Trip Health, providing cutting edge mental health care in Toronto, Montreal, Vancouver, Ottawa, Hamilton, Kitchener-Waterloo, Thunder Bay, Sault Ste. Marie, and at-home.